Computational and theoretical chemistry
Led by Patricia Hunt, this research group uses the tools of chemistry to understand how molecules are made and broken.
Our research is focused on understanding the chemistry and physics associated with solvents and solvation, particularly as this applies to ionic-liquids, molten salts, and deep eutectic solvents.
We study the making and breaking of molecules. This includes catalytic mechanisms (for group II and frustriated lewis acid-base pairs) and chemical decomposition (for green fuels, bio-fuels, and ionic-liquids).
Spanning all of these areas is a specialisation in hydrogen-bonding and acid-base interactions, and expertise in the MO theory of bonding, non-covalent interactions, and charge partitioning within molecules.
Find out more about the Hunt reserarch group.
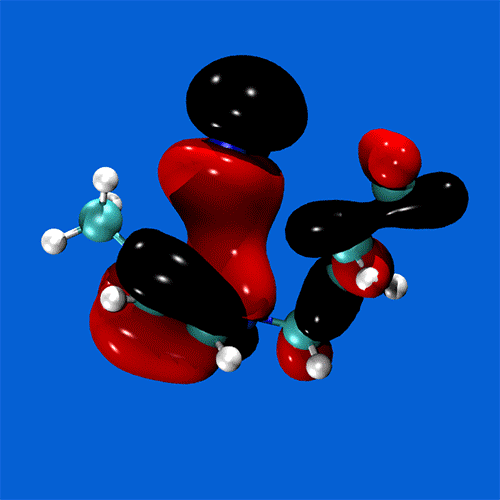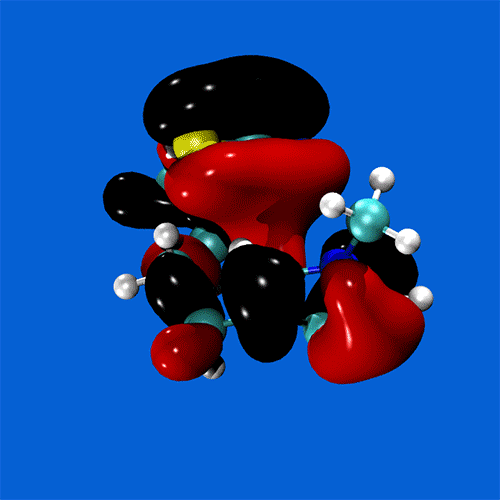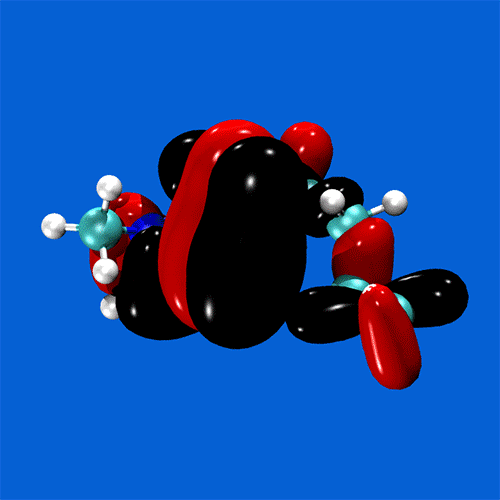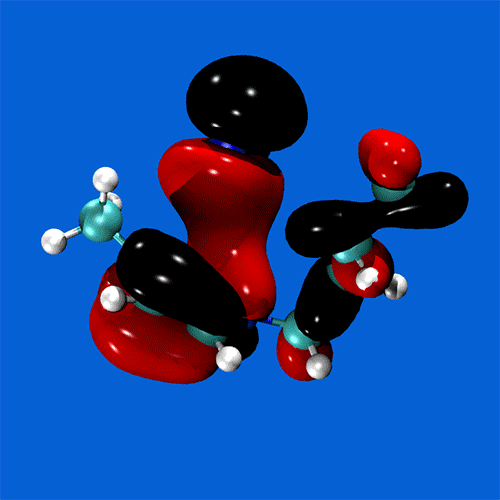
Research staff
Professor
School of Chemical and Physical Sciences
Publications
- ‘Bi(III) halometallate ionic liquids: Interactions and speciation’, R. Rowe, K Lovelock and P. A. Hunt JCP, 2021, 155, no. 014501.
- ‘Conformational design concepts for anions in ionic liquids’, F. Philippi, D. Pugh, D. Rauber, T. Welton and P. A. Hunt, Chemical Science, 2020, 11(25), pp 6405–6422.
- ‘H-bonding in ionic liquids’, P.A. Hunt, R. Matthews and C. Ashworth, Chemical Society Reviews, 2015, 44, pp 1257–1288
- ‘Hydrophosphination of styrene and polymerisation of vinylpyridine: A computational investigation of calcium catalysed reactions and the role of fluxional non-covalent interactions’, B. Ward and P.A. Hunt ACS-Catalysis, 2017, Vol 7 (1), pp 459–468

