Dissolving a myth about chemical solutions
A Victoria University study highlights a crucial but often overlooked consideration during sample preparation involving dilutions - a procedure at the heart of many chemistry experiments.
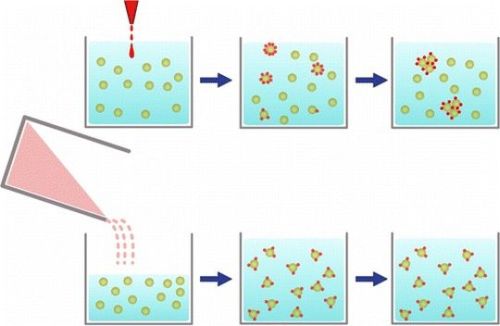
Dilutions are used in numerous contexts, from chemical synthesis to biochemistry, to prepare samples of given concentrations. When adequate precautions are taken, it is generally assumed that the exact dilution steps do not affect the final product.
Research by Dr Eric Le Ru and his PhD student Brendan Darby, from the School of Chemical and Physical Sciences and the MacDiarmid Institute for Advanced Materials and Nanotechnology, demonstrates that this simple assumption is not always correct in samples containing nanoparticles.
Dr Le Ru says the competition between adsorption dynamics and diffusion may result in extreme variations in molecule concentrations across a solution, especially in cases where large dilution factors are used.
"Such non-uniformity is clearly undesirable and can in many contexts have dramatic consequences on the experimental outcome and/or their interpretation. We used a technique called surface-enhanced Raman spectroscopy (SERS) to demonstrate this effect," he says.
This study identifies a common but unnoticed source of irreproducibility and fluctuations due to sample preparation.
"Our work should be a warning call for any area of research in chemistry where molecular surface adsorption plays an important role, for example catalysis (used for chemical synthesis) or super-capacitors (used in batteries)," says Dr Le Ru.
Read the full journal article online: http://pubs.acs.org/doi/abs/10.1021/ja506361d
