Research projects
Find out about postgraduate research being conducted in the field of Organic Synthesis.
PhD, Masters and graduate research projects are available in total synthesis of natural products, synthesis of natural product analogues, structure-based drug design, and synthetic methodology.
Currently available projects
6-(2-Hydroxyethyl)azulene as a coloured protecting group
Azulene (and its alkyl derivatives) is an intensely blue-coloured organic molecule. It has properties that are distinct from those of other organic dyes and has found many applications. We have recently shown that 6-(2-hydroxyethyl) azulene is a protecting group for carboxylic acids. This project would extend its use into amino acid protection, which could have applications in peptide synthesis.
Further projects are available, dependent on student interest, that will investigate incorporation of alternative azulene species into organic materials.
Background reading
Azulene-Based Protecting Groups, PhD thesis of former student Thomas Bevan, 2016.
A colourful azulene-based protecting group for carboxylic acids, T. W. Bevan, J. Francis-Taylor, H. Wong, P. T. Northcote, J. E. Harvey. Tetrahedron, 2018, 74, 2942. https://www.sciencedirect.com/science/article/pii/S0040402018304708
Structure-based design of kinase inhibitors
Kinases are required for many cellular processes, including signalling leading to proliferation, migration, calcium regulation. They represent important targets for modern drug design. Current PhD student Jordan McCone has been developing a computational model of Bruton’s tyrosine kinase (Btk) for a natural product-inspired drug design project.
Available projects can be tailored to suit the background of the student, including developing models of other therapeutically relevant kinases (more docking and virtual screening emphasis) or using our prepared Btk model to discover new drugs (will involve some docking/virtual screening but also synthesis of new drug lead candidates).
Pest Pheromone Synthesis
New Zealand’s ecological niche is constantly under threat from introduced pests, while agricultural pests cause great economic harm both locally and globally. Synthesis of pheromones (and analogues) enables us to use selective ways to target pests.
Synthesis of Risperidone Analogues
Risperidone is an anti-psychotic drug used to treat schizophrenia. It also displays immunomodulatory characteristics. With the goal of exploring how changes in the linker length affect activity, we are developing analogues of risperidone for immunological studies.
Scalable synthesis of peloruside A
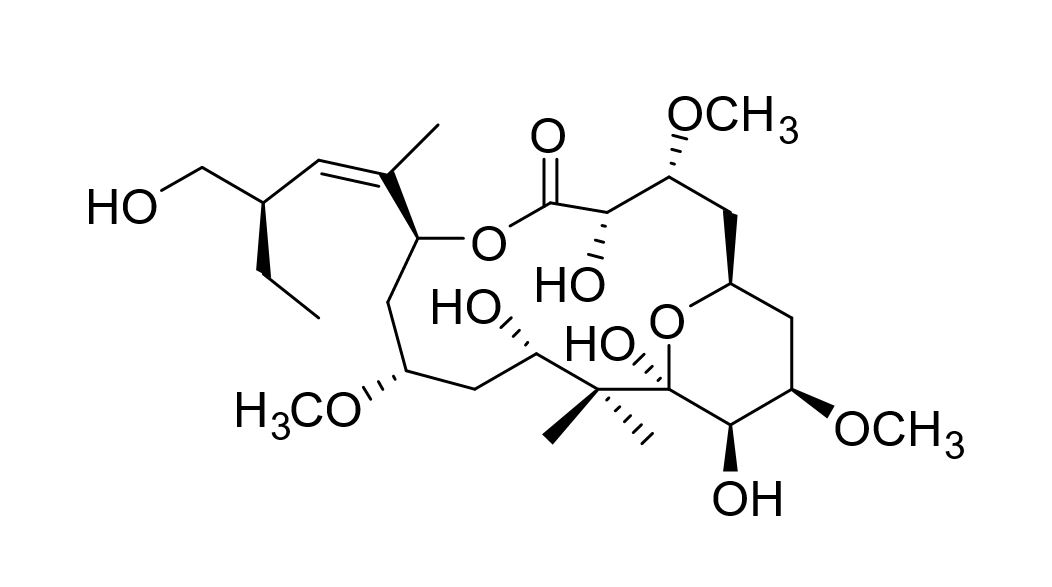
Peloruside A is a potently cytotoxic sponge natural product discovered by Northcote and co-workers. It has better physical properties than cancer drugs on the market. We are developing a scalable synthesis of peloruside A to enable preclinical studies and a Masters or PhD project is available to continue this work.
Background reading
Developing a scalable synthesis of Peloruside A, Amira Brackovic PhD thesis, 2019. http://researcharchive.vuw.ac.nz/handle/10063/7845
Synthesis and stereochemical elucidation of rimarikiamide
Rimarikiamide was isolated recently by Dr Rob Keyzer’s research group at Victoria University of Wellington. The unusual presence of a taurine residue and uncertain stereochemical configuration have prompted us to pursue its synthesis, with also the added benefit that we can access the other stereoisomers and evaluate their bioactivity.
Background reading
Toward the Synthesis of Rimarikiamide A, Ash Asbury Masters Thesis, 2017. https://viewer.waireto.victoria.ac.nz/client/viewer/IE989542/details?dps_dvs=1547600059291~881
Synthesis of “zampanalogues”
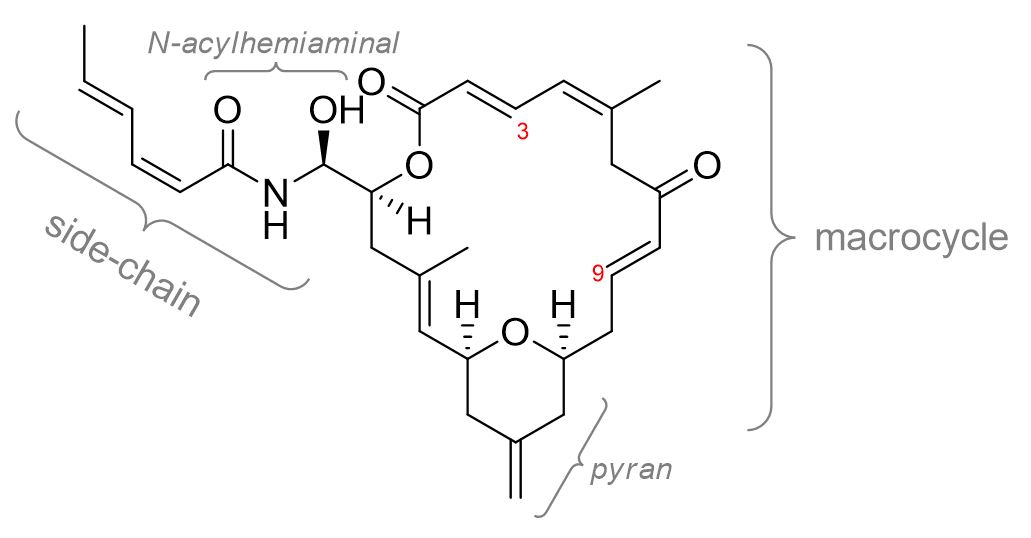
Zampanolide is an anti-cancer natural product from marine sponges that is a potent inhibitor of cancer cell growth (IC50 = 1–5 ng/mL for the P388, HT29, A549 and MEL28 cell lines). It binds covalently to b-tubulin, a protein that is part of the microtubule structure.
Zampanolide has improved physical properties over other cancer drugs and is not susceptible to multidrug resistance, which provides benefits over existing treatments.
This project aims to synthesise compounds that are structurally related to zampanolide (analogues) based on the recently published crystal structure of zampanolide in its binding site on b-tubulin. In particular, we have identified regions of the side-chain that could be modified to improve the strength of the binding and therefore provide greater potency or better selectivity for tubulin compared to other proteins.
Background reading
Chasing "Zampanalogs": Advancing the Synthesis of the Zampanolide Macrocycle, Sophie Geyrhofer PhD thesis, 2017. http://researcharchive.vuw.ac.nz/handle/10063/7021
Synthesis of peloruside A analogues

Peloruside A is a potently cytotoxic sponge natural product discovered by Northcote and co-workers. It has better physical properties than cancer drugs on the market. PhD projects are available for developing novel analogues of peloruside A.
Background reading
Synthetic, Semisynthetic and Natural Analogues of Peloruside A. Amira Brackovic and Joanne E. Harvey*, Chem. Commun., 2015, 51, 4750–4765. DOI: 10.1039/c4cc09785h.
Synthesis of pateamine A analogues
Pateamine A is a New Zealand marine sponge natural product with potent cytotoxicity and interestingly selective and finely tuned inhibition of protein translation. In collaboration with A.Prof. Paul Teesdale-Spittle (link https://wgtn.ac.nz/sbs/about/staff/paul-teesdale-spittle), we are exploring the synthesis of simplified analogues of pateamine for the study of these biological properties.
Background reading
Synthesis of a simplified triazole analogue of pateamine A. A. Hemi Cumming, Sarah L. Brown, Xu Tao, Claire Cuyamendous, Jessica J. Field, John H. Miller, Joanne E. Harvey and Paul H. Teesdale-Spittle*, Org. Biomol. Chem. 2016, 14, 5117–5127. DOI: 10.1039/c6ob00086j
Synthesis of furopyranone fungal natural products
We have been pursuing the synthesis of the fungal natural product TAN-2483B and developing analogues. The latter have interesting bioactivity that prompt further research into analogue molecules of the furo[3,4-b]pyran-5-one natural products.
Background reading
Synthesis of Bioactive Side-Chain Analogues of TAN-2483B, Kalpani K. Somarathne,[a] Jordan A. J. McCone,[a,b] Amira Brackovic,[a] José Luis Pinedo Rivera,[a] J. Robin Fulton,[a] Euan Russell,[c] Jessica J. Field,[c] Christopher L. Orme,[a] Hedley L. Stirrat,[a] Jasmin Riesterer,[a] Paul H. Teesdale-Spittle,[b,c] John H. Miller,[c] and Joanne E. Harvey*[a,b], Chem. Asian J. 2019, 14, 1230–1237. DOI: 10.1002/asia.201801767. https://onlinelibrary.wiley.com/doi/abs/10.1002/asia.201801767 Synthesis of labillarides E-H
Colleagues at Victoria University have isolated a family of unusual oxylipin natural products, labillarides E-H. We have developed a synthetic method for producing the constituent furo[3,2-c]pyran-4-one core. Extending this to the synthesis of the natural products is the aim of this project.
Background reading
Transition Metal Catalyzed Linchpin-Based Strategies in Natural Product Synthesis: Synthesis of Asteriscunolide D, Aspergillide B and the Core of Labillarides E-H, Mark Bartlett PhD thesis, 2013. https://viewer.waireto.victoria.ac.nz/client/viewer/IE168054/details?dps_dvs=1547600445507~269

